The Annual Reporting by Prescription Drug Wholesale Distributors and 4 Third-Party Logistics Providers is new reality for industry. This means that if you are pharmaceutical wholesaler and a 3PL you must submit your company information to the FDA. The actual FDA guidance:
Wholesale Distributor Annual Reporting
The Annual Reporting by Prescription Drug Wholesale Distributors and 4 Third-Party Logistics Providers is new reality for industry. This means that if you are pharmaceutical wholesaler and a 3PL you must submit your company information to the FDA. The actual FDA guidance:
This guidance describes FDA’s expectations for prescription drug wholesale distributors (wholesale distributors) and third-party logistics providers (3PLs) for the annual reporting to FDA as required under the Drug Supply Chain Security Act of 2013 (DSCSA). Under section 584(b) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. 360eee-3(b)), beginning November 27, 2014, 3PLs must report certain information to FDA, including State licensure information for each facility and the name and address for each facility. Under section 503(e)(2)(A) (21 U.S.C. 353(e)(2)(A) (as amended by the DSCSA), beginning January 1, 2015, wholesale distributors also must report certain information to FDA, including State licensure information for each facility, contact information for each facility, and any significant disciplinary actions taken by a State or the Federal Government. This guidance outlines the information that should be submitted to FDA, the timing of the submissions, a preferred format 28 for the submissions, and a preferred method for reporting to FDA.
What needs to be submitted:
The DSCSA requires contact information to be submitted by wholesale distributors. FDA considers contact information to include the email address and telephone number of the person who will interact with the FDA. In addition to the specific information required by DSCSA to be submitted to the Agency, FDA has identified additional information that will enhance efficiencies and improve accuracy in the management of the licensing and facility information submitted to the Agency. Therefore, FDA is requesting that certain additional information be submitted to FDA on a voluntary basis. This additional information will be useful to FDA in its enforcement of the Act and to stakeholders as they make decisions about their drug product distribution. Furthermore, FDA is requesting the same information from wholesale distributors and 3PLs. The ultimate goal is for the public database to serve as a single repository of licensing and facility information for wholesale drug distributors and 3PLs conducting business in the United States.
Where do you need to go to submit this information?
If you are a client of TrackTraceRx, we assist you in this submittal. If not please look at these links:
The actual form:
https://direct.fda.gov/apex/f?p=100:LOGIN_DESKTOP:2674113566440:::::
To register for the form:
https://direct.fda.gov/apex/f?p=100:5:105890746929503::NO:::
When should you fill out this information?
Wholesale distributors: Immediately
What about for newly registered company?
Wholesale distributor and 3PL facilities that are newly licensed after the dates noted above should initially report within 30 days of obtaining a State or Federal license.
When do I have to re-submit my information on an annual basis?
Wholesale distributors: annually
What else?
Reports of significant disciplinary actions should be submitted to FDA when a final action or ruling has been made by a State or Federal licensing authority.
Wholesale distributors: within 30 days of final action
A company should notify FDA if a facility goes out of business or decides to voluntarily withdraw a State or Federal license.
“ FDA prefers the use of extensible markup language (XML) files in a standard Structured Product 268 Labeling (SPL)4 format.”
How do i submit this report?
FDA prefers the use of extensible markup language (XML) files in a standard Structured Product 268 Labeling (SPL)4 format.
or
Register and fill out the form here:
https://direct.fda.gov/apex/f?p=100:LOGIN_DESKTOP:2674113566440:::::
Step-by-Step Guide
Step 1.
Visit the login portal: https://direct.fda.gov/apex/f?p=100:LOGIN_DESKTOP:17509663700106:::::
Below you will see the FDA CDER portal. (click to enlarge) Chances are you probably won’t have an account yet. Click on the Create Account link.
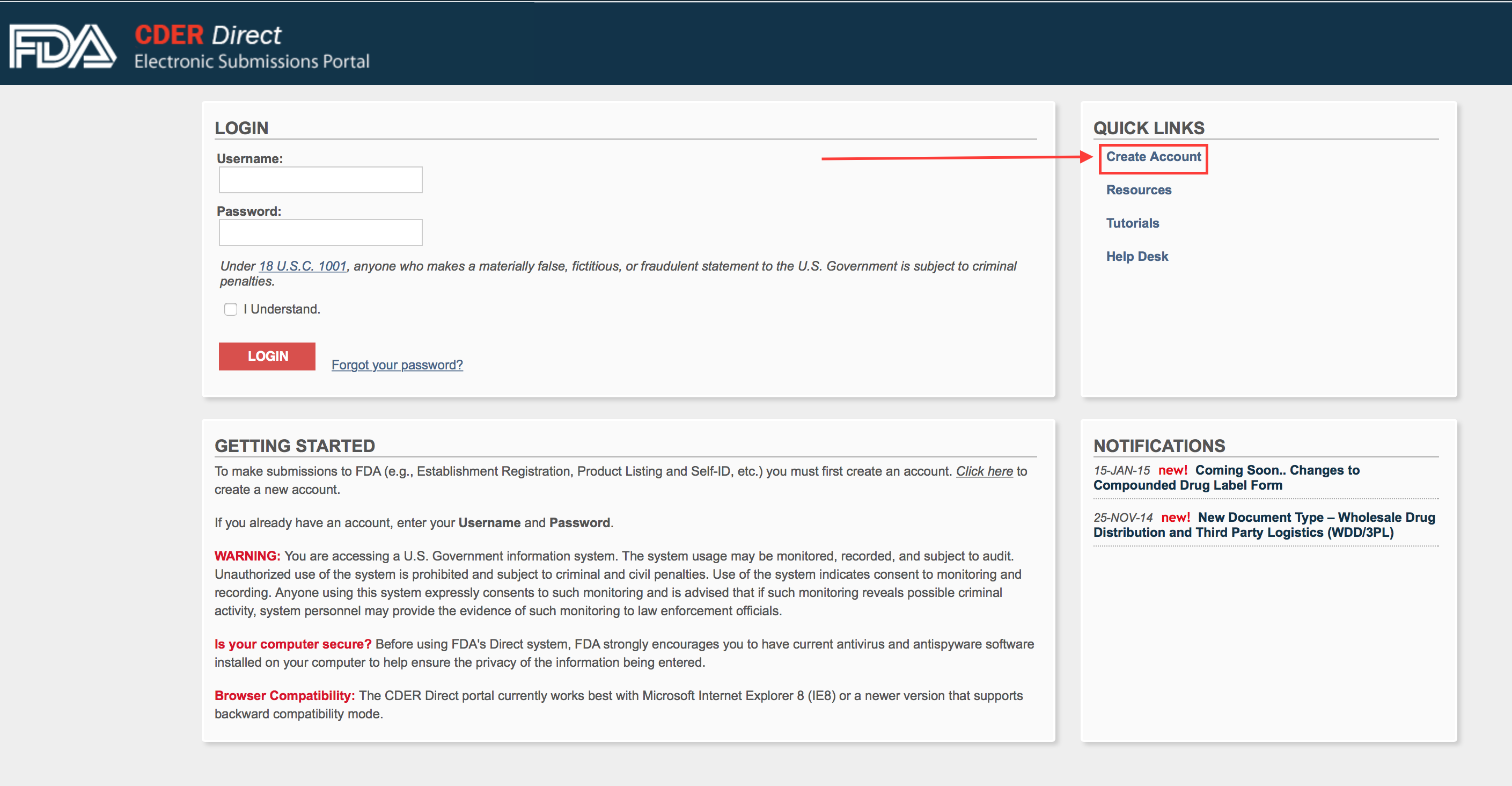
Step 2.
Register for an account. https://direct.fda.gov/apex/f?p=100:5:17509663700106::NO:::
Fill out your company information that is appropriate. You will need a DUNS number to proceed. You can get a free DUNS number at http://dnb.com/. Select the WDD/3PL check box and click submit. Momentarily the FDA will send you your CDER number and credentials on your email.
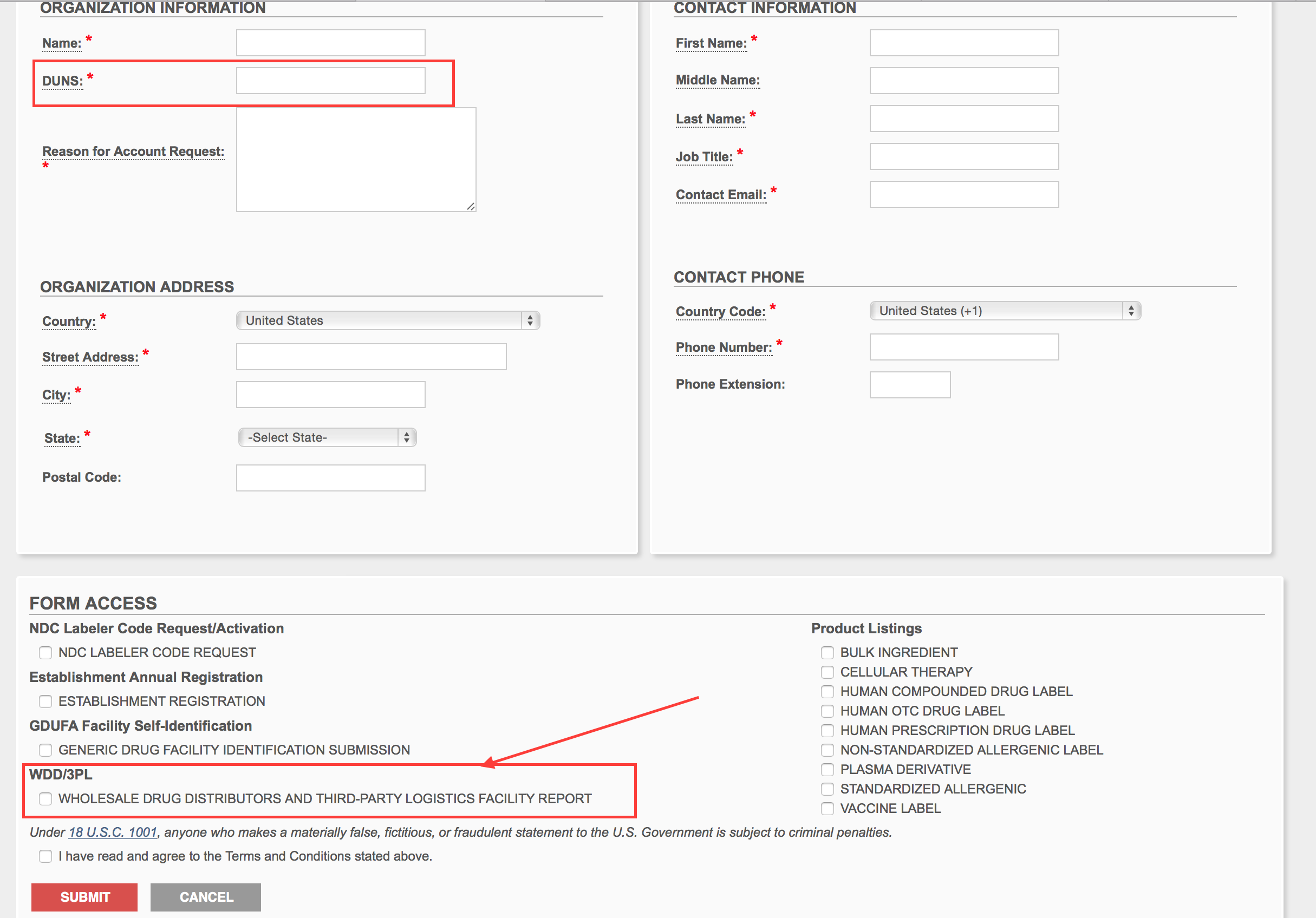
Step 3.
Visit again the login portal: https://direct.fda.gov/apex/f?p=100:LOGIN_DESKTOP:17509663700106:::::
Type in your credentials and hit login.
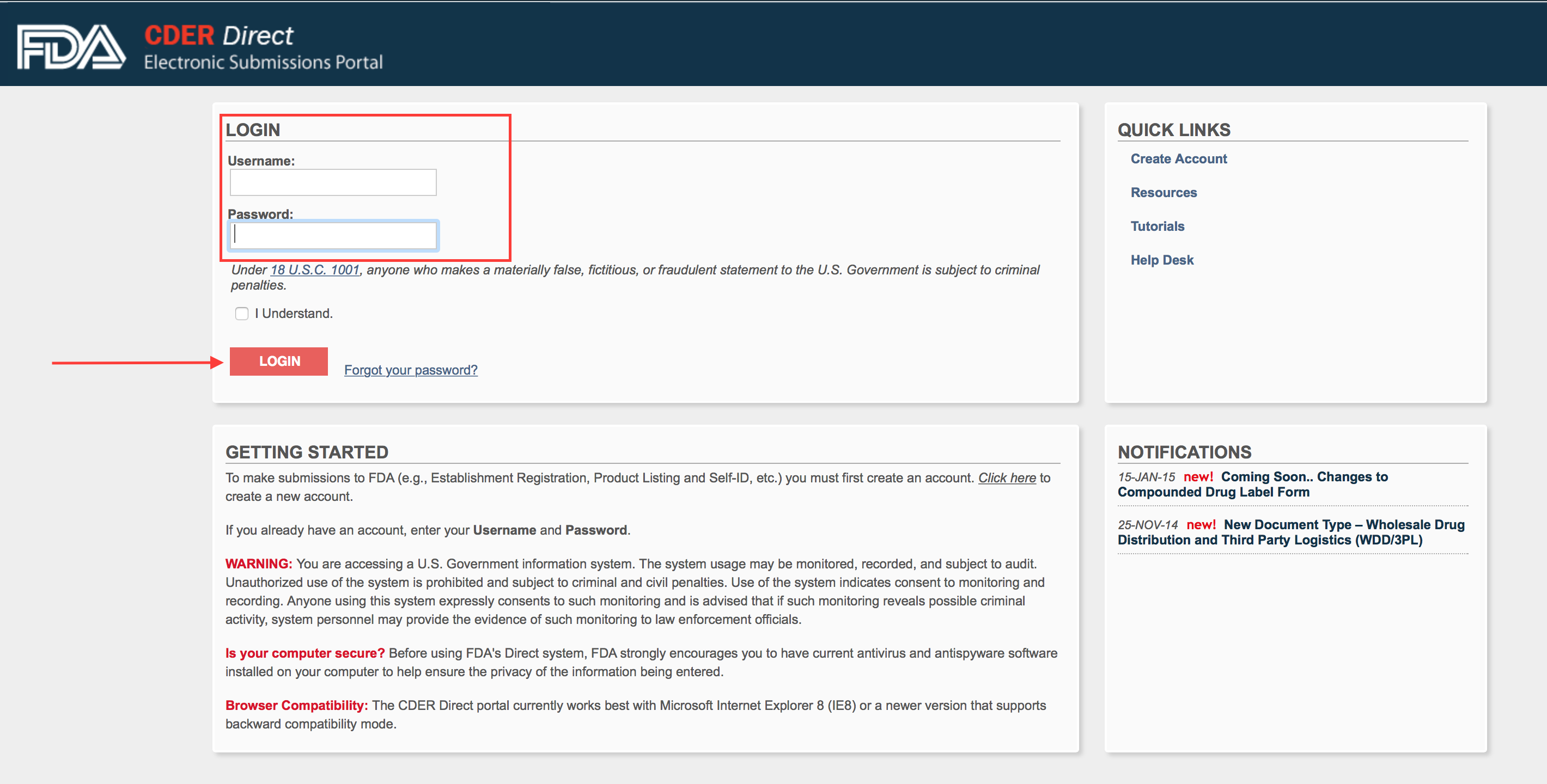
Step 4.
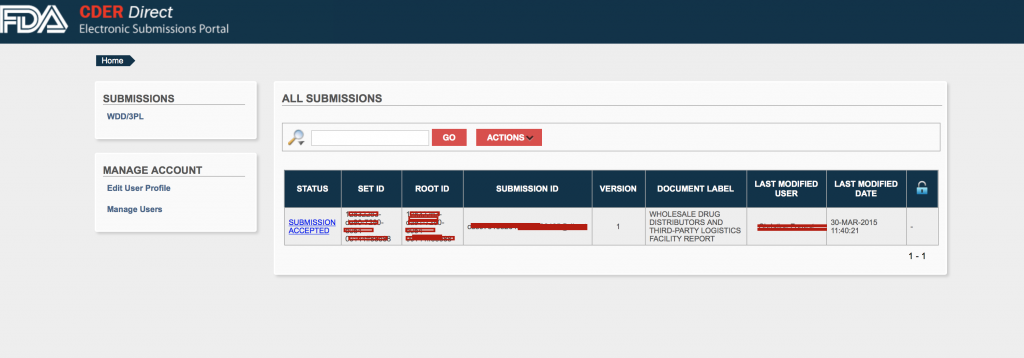
Once logged in, you will be able to see any previous submittal. Click on the WDD/3PL link.
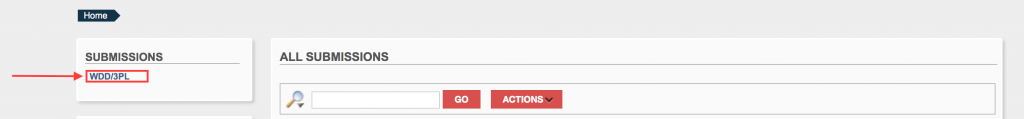
Step 5.
Next, you will select “Create a new WDD/3PL using a blank form. Click “continue”.
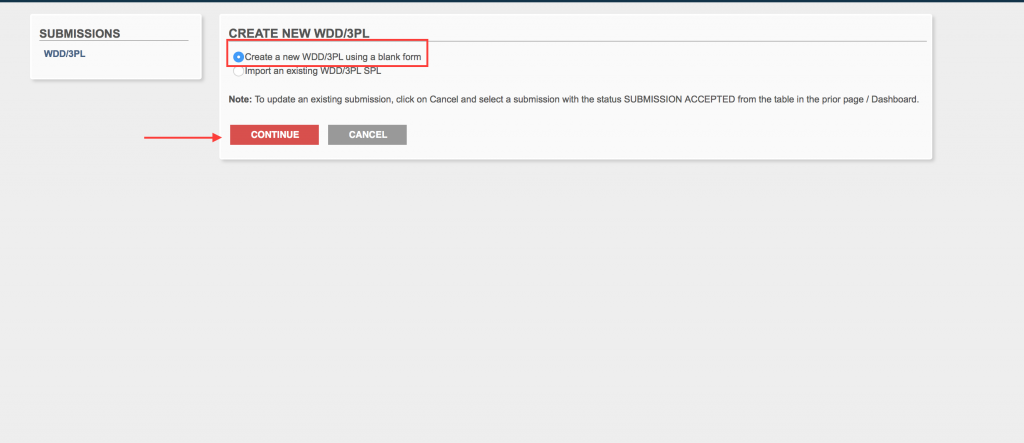
Step 6.
You will now be in the SPL submission section. Fill out the appropriate information and click “Save Draft”. Once a draft is saved, you need to add a facility. Click the “Add a Facility” button in the bottom.
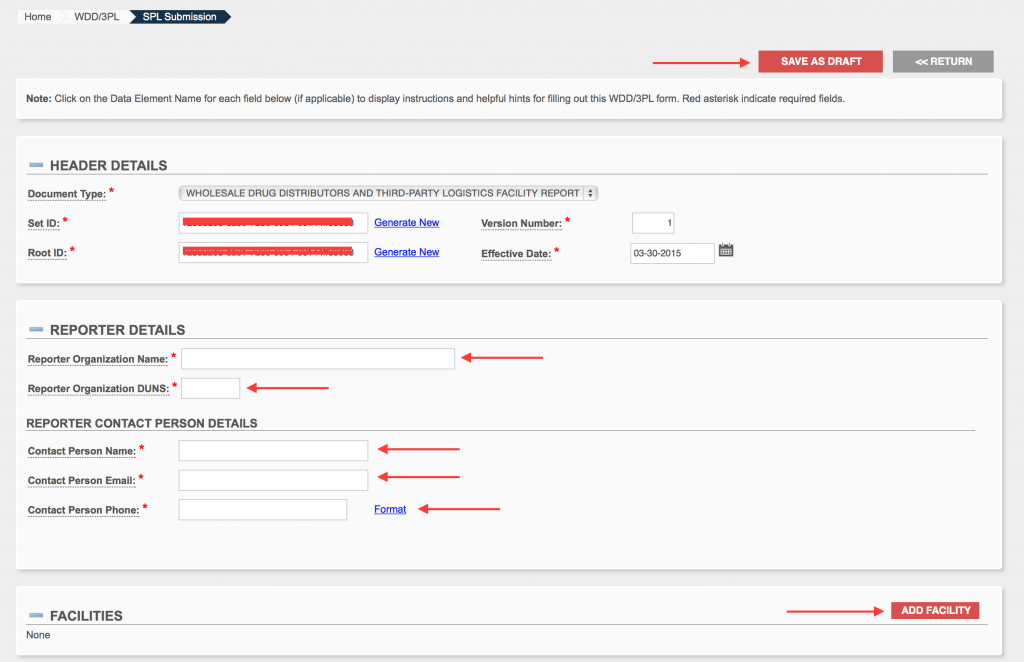
Step 7.
You will now be able to add multiple of your facilities. Fill out the appropriate information. Add any different trade names per facility and select if it is a WDD or 3pl. Click “Save Facility” at the top right.
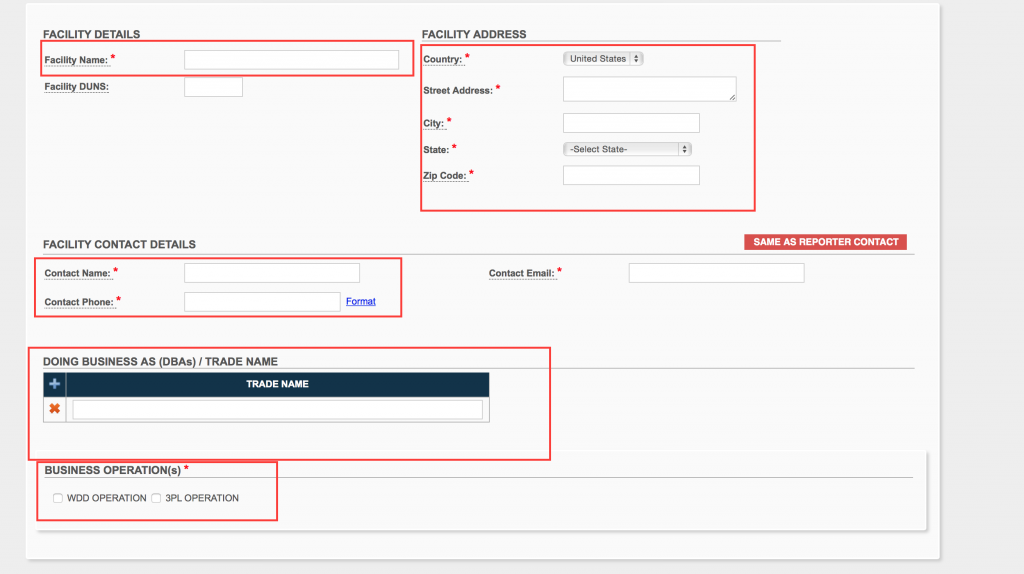
Step 8.
Once the facility has been saved, you can add additional ones or click “Submit SPL”.
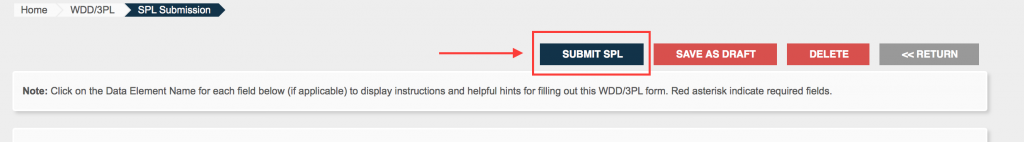
Step 9.
Once submitted, you should see your annual report submittal.
Free VRS Evaluation
With the deadline fast approaching, contact TrackTraceRx today to receive a free evaluation of your VRS strategy to comply with the DSCSA Saleable Returns. This free consultation will allow you to have a piece of mind that you are following the correct procedures in order to meet ALL DSCSA requirements. TrackTraceRx will also provide you with a FREE Standard Operating Procedure (SOP) template which is required by the DSCSA during a FDA inspection.




